
TOKYO, Aug 8, 2023 – (JCN Newswire via SEAPRWire.com) – Aichi Cancer Center and NEC Corporation’s research group with Gifu University, Toyama University and Kitasato University Medical Center, have developed a method for efficiently identifying the lung cancer antigens and the antigen-specific T cells that recognize the antigens through both a single-cell analysis of Tumor Infiltrating Lymphocytes (TILs) and NEC’s AI-based antigen prediction system that predicts immune response. Our paper describing the results of this study was published on August 6, 2023 in the “Journal for ImmunoTherapy of Cancer,” which is the official journal of the Society of Immunotherapy of Cancer (SITC) in the United States.
 |
 |
 |
 |
Research Background
Lung cancer is one of the most common cancers and one of the leading causes of cancer death worldwide. There are many types of cancer treatment, such as surgery, chemotherapy, radiation therapy, molecular targeted therapy, immunotherapy, and combinations of these. Recently developed immune checkpoint inhibitors (ICI) have attracted attention as a new therapy, and lung cancer is one of the most sensitive cancers to ICI, but it is effective in only a subset of individuals. Accordingly, new effective immunotherapies are required for lung cancer.
Cytotoxic T lymphocytes (CTLs) in TIL are crucial immune cells that can specifically recognize and eliminate tumor cells. Antigens targeted by CTL include patient-specific neoantigens and common antigens commonly expressed among patients such as cancer-testis antigens (CTA). In general, it is not easy to identify any antigens. If these antigens can be efficiently identified, a combination therapy with ICIs and antigen-specific immunotherapy may enhance the efficacy of treatment.
Contents and results of this research
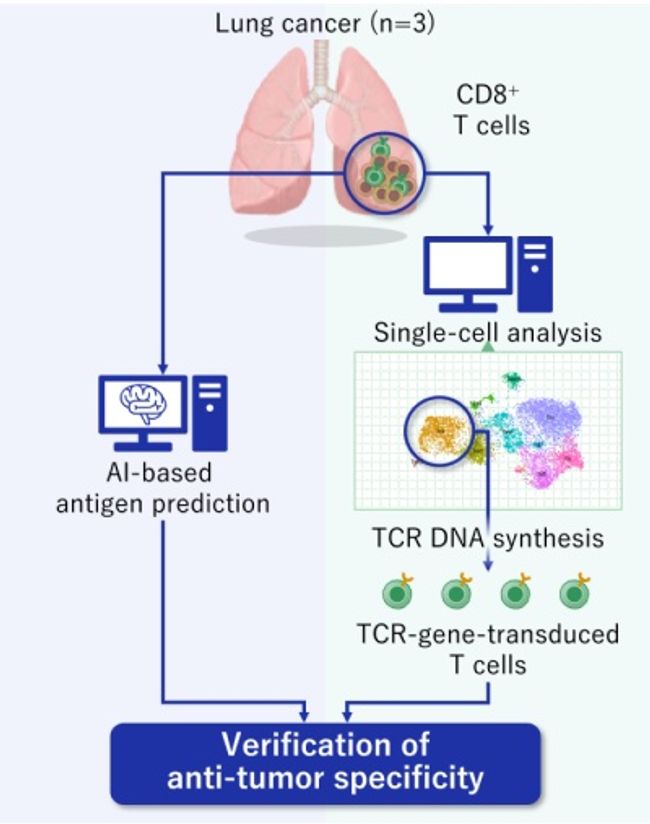
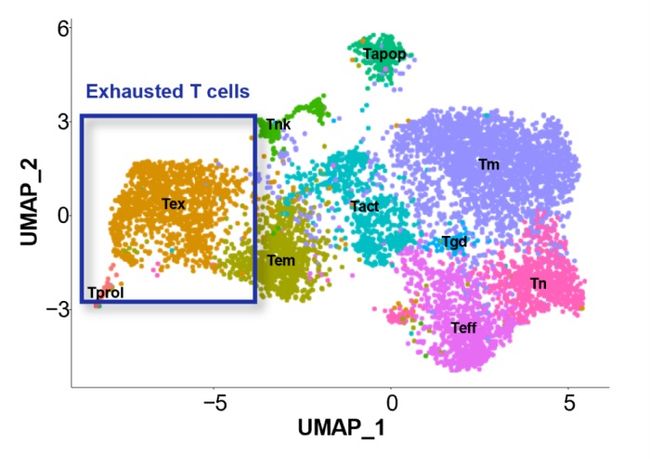
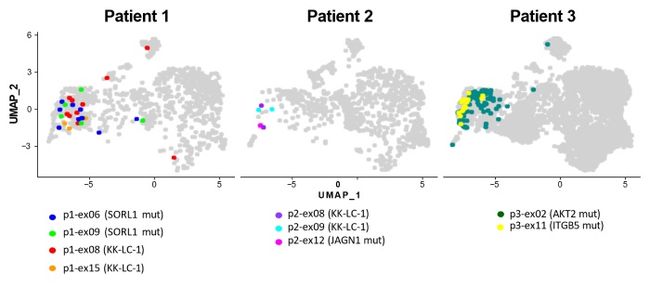
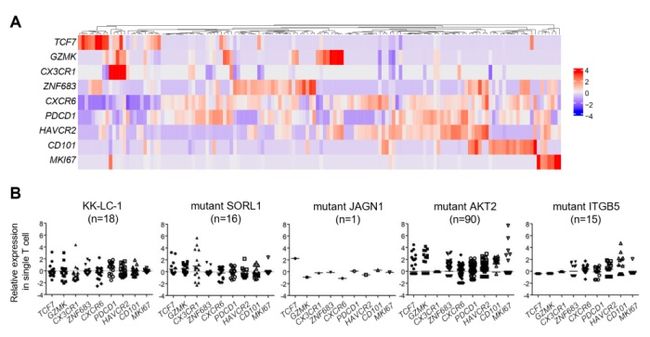
In this study by Aichi Cancer Center and NEC, we performed a single-cell analysis to determine the TILs characteristics of patients with surgically resected non-small cell lung cancer (NSCLC) (n=3) (Figure. 1). Then, we divided the TILs into 10 clusters based on gene expression profile, and identified the exhausted T cell cluster (Tex cluster) characterized by the expression of the genes called exhaustion markers (Figure. 2). We synthesized the TCRs contained in the identified exhausted T cell cluster and induced each of the TCRs into each corresponding T cell, and examined the immune responses to neoantigens predicted by NEC’s AI-based antigen prediction system and typical CTAs. It was confirmed that NEC’s AI-based antigen prediction system can accurately predict the antigens that cause the immune responses, and we identified four TCRs recognizing KK-LC-1 (one of the CTAs, *2), and five TCRs recognizing the neoantigens (Figure. 3).
Researcher’s comment
Dr. Hirokazu Matsushita, Chief, Division of Translational Oncoimmunology, Aichi Cancer Center
In collaboration with NEC and leading research institutes, the Aichi Cancer Center has developed a procedure for efficiently identifying antigens and antigen-specific T cells using surgical samples from patients with cancer. Looking ahead, we will add a spatial analysis of the cancer microenvironment that contains antigens and antigen-specific T-cells to this system to further clarify the nature of the T-cells infiltrating the cancer. In addition to aiming to develop innovative cancer immunotherapies from these studies, we will apply the knowledge gained to other cancers as well.
Yoshiko Yamashita, Ph.D. Senior Professional, AI Drug Development Division, NEC Corporation
NEC is conducting clinical trials of personalized cancer vaccine therapies targeting neoantigens. We believe that we have taken a step forward in realizing even more sophisticated personalized cancer vaccine immunotherapies and engineered T cell therapies by using a method to identify antigen-specific T cells constructed in this study and an Attentive Variational Information Bottleneck (AVIB) method (*3) to predict interactions between TCRs and antigens using AI newly developed by NEC. We will continue to accelerate research and development to provide effective treatments to patients.
Research support
Aichi Cancer Center Priority Project Research
NEC Corporation
Subsidy Program for Scientific Research by the Japan Society for the Promotion of Science
Japan Respiratory Foundation
Uehara Memorial Life Science Foundation
(1) Single-cell analysis is an analysis method that is capable of detecting RNA, which is a DNA transfer product for each cell, rather than as a mass of tissue, and understanding the individuality and diversity of individual cells based on the level of gene expression.
(2) KK-LC-1 (KitaKyushu Lung Canner antigen-1): Cancer and testis antigens that have been reported to be expressed in cancer.
(3) Attentive Variational Information Bottleneck (AVIB) Method: Variation information bottleneck method developed by NEC Laboratories Europe and NEC Laboratories America to predict interactions between TCRs and antigens using AI. (bit.ly/43YKys6)
About NEC Corporation
NEC Corporation has established itself as a leader in the integration of IT and network technologies while promoting the brand statement of “Orchestrating a brighter world.” NEC enables businesses and communities to adapt to rapid changes taking place in both society and the market as it provides for the social values of safety, security, fairness and efficiency to promote a more sustainable world where everyone has the chance to reach their full potential. For more information, visit NEC at www.nec.com.
Copyright 2023 JCN Newswire. All rights reserved. (via SEAPRWire)

